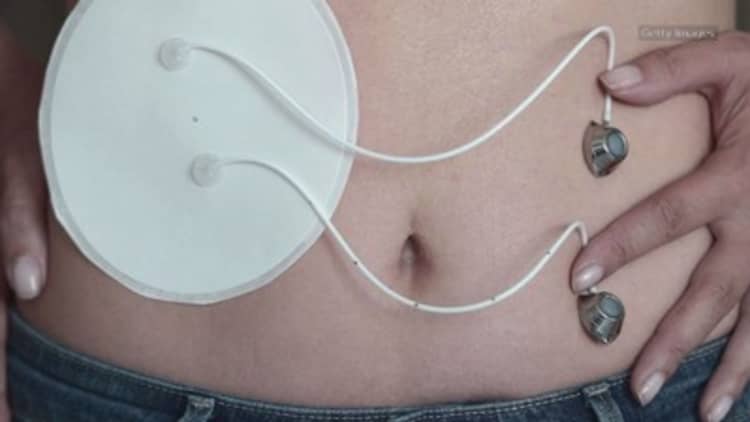
For people with type 1 diabetes, life is a perpetual tightrope act. They must carefully monitor the dosage and timing of insulin injections that allow them to teeter on the high wire that is optimum glucose control. Too little insulin, and their glucose levels rise, leaving them at risk over time for complications such as blindness and kidney failure; too much insulin and hypoglycemia (low blood glucose) results, making them vulnerable to coma or, in extreme circumstances, even death.
Now researchers studying type 1 diabetes believe they have found a way to help patients avoid the tightrope walk altogether. Several academic and commercial groups are conducting clinical trials for the latest generation of what's known as the artificial pancreas. Contrary to what the name might suggest, artificial pancreas systems involve no transfer of tissue. Rather, the term refers to a complex technology that uses computer algorithms to automatically and continuously sense a person's unique blood glucose balance and then substitute the endocrine function of a healthy pancreas.
The new technology is a part of what the President's Council of Advisors on Science and Technology refer to as personalized medicine — which the organization defines as "the tailoring of medical treatment to the individual characteristics of each patient."
One company at the forefront of this approach is Dublin-based Medtronic, which estimates that its advanced version of an artificial pancreas system could be in the marketplace by next year. Meanwhile, a team of researchers led by Dr. Boris Kovatchev, director of the University of Virginia Center for Diabetes Technology, has announced that the group will begin final clinical trials this summer on another artificial pancreas system developed by the University and refined for clinical use by the start-up company TypeZero Technologies.
Still other research programs are in progress at Cambridge University and also at Boston University, where a team led by Professor Edward Damiano is developing a product that will measure both insulin and glucagon, a peptide hormone that works to raise the concentration of glucose in the bloodstream.
Dr. Marc Breton, associate professor at UVA's Center for Diabetes Technology, says the technology behind artificial pancreas systems is being improved and refined daily. "The algorithms it is based on are resulting in better and better outcomes. For people with type I diabetes, it's quite a hopeful time."
New hope for millions
A key player in the development of the artificial pancreas was JDRF, previously known as the Juvenile Diabetes Research Foundation. Several years ago, despite awareness that artificial pancreas technology could be helpful for type 1 diabetes, there was little interest from the industry to develop the product. JDRF's chief mission officer, Aaron Kowalski, Ph.D., stepped in to persuade industry and regulatory officials that the technology was worthwhile and could be successfully marketed. The organization has since funded millions of dollars' worth of research in the field.
The hope is that the new systems can transform the lives of the estimated 1.25 million U.S. individuals who suffer from type 1 diabetes and the estimated 80,000 children around the globe who are diagnosed each year. Type 1 diabetes is typically diagnosed in childhood or adolescence, requiring patients, who can't produce their own insulin, to depend on daily injections or a conventional insulin pump.
In children, this can be especially worrisome, because without constant monitoring, dangerously low glucose levels may go undetected in the overnight hours. Parents typically must awaken several times during the night to test their children's levels and then be prepared to intervene on a moment's notice.
Artificial pancreas systems hope to eliminate this danger by taking existing pump technology to a new level. While most earlier versions primarily served as blood glucose monitors, the newest technology goes a step further. It is the force behind what scientists have come to call a "closed-loop" system, consisting of a pump worn outside the body, a continuous glucose monitor, which measures glucose from fluid under the skin, and a device that runs continuous algorithms to determine how much insulin should be delivered.
The devices "close the loop" by providing not only continuous monitoring of blood glucose but continuous adjustments in the amount of insulin supplied through the pump.
Xavier Hames, a four-year-old Australian boy with type 1 diabetes, was the first person to use a precusor to Medtronic's new system. His mother, Naomi Hames, said it helped ease the family's daily worries about their child's fluctuating glucose levels. "Having this new system gives us the reassurance that Xavier is safe when we are all asleep at night and during the day," she said.
Two final clinical trials, funded by the National Institutes of Health, will test UVA's product with larger groups of people in locations throughout the United States and Europe beginning this summer. The trials will be conducted at eight test sites. In addition to UVA, they include Harvard University, Mount Sinai School of Medicine, Mayo Clinic, University of Colorado, Stanford University, University of Montpellier in France, University of Padova in Italy and the Academic Medical Center at the University of Amsterdam in The Netherlands.
In the first study, 240 patients with type 1 diabetes will use the artificial pancreas for six months while participating in their regular daily routines. The device will be compared with a standard insulin pump on two metrics: whether it reduces the risk of hypoglycemia, or low blood sugar, and how well it holds blood-glucose levels in check.
In the second study, 180 patients who have completed the first study will undergo an additional six months of testing. That group will test a Harvard University algorithm to determine the degree of blood glucose control in patients.
Until the clinical trials are complete, the question of how safe and effective the devices will be over the long term remains unanswered.
Moreover, the U.S. Food and Drug Administration and other regulatory groups would have to confer a final seal of approval before the new devices could be marketed for use by patients.
But expectations remain high, especially among the millions of children and adults for whom the requirements and uncertainties of type 1 diabetes steal away precious minutes of each day.
Said Julia Greenstein, JDRF's vice president of Discovery Research, "Our constituents are very excited about these new avenues of research and will be watching very carefully to see all the trials unfolding."
— By Annetta Miller, special to CNBC.com
Correction: This article has been updated to reflect that patients with Type 1 diabetes are unable to produce insulin.






