Every year in Chicago, tens of thousands of people with a stake in cancer research gather in the nation's largest convention center to learn about the latest developments in treating the disease.
The updates at the American Society of Clinical Oncology conference can be practice-changing for doctors, life-changing for patients, and — for investors — market-moving.
Many of the most stock-moving results were released over the weekend. Here are five takeaways.
Merck retains lead in lung cancer
"#ASCO18 is about lung cancer treatment," Hal Burstein of the Dana-Farber Cancer Institute, wrote on Twitter on Saturday.
And the winning drug for treating lung cancer, the leading cause of cancer-related deaths and the biggest market for cancer drugs, continues to be Merck's immunotherapy Keytruda.
In results presented over the weekend, Merck's drug was shown to be more effective as an initial treatment for patients with advanced nonsmall cell lung cancer, the most common form of the deadly disease, than chemotherapy.
A study in patients whose cancer expressed a biomarker known as PD-L1 showed that those treated first with Keytruda lived a median four to eight months longer than those on chemotherapy. What's more, they experienced fewer onerous side effects.
"I view this as a double win for patients," John Heymach, an ASCO expert with the MD Anderson Cancer Center, said in a media briefing Sunday. "Not only are patients living longer ... but they're also receiving a treatment that has substantially less toxicity."
Another Keytruda study, in squamous nonsmall cell lung cancer — which accounts for 25 to 30 percent of cases – "should establish the Keytruda/chemo combo as the new standard of care," Jefferies analyst Ian Hilliker said in a Sunday research note.
Merck's drug competes with others, such as by Bristol-Myers Squibb and Roche. Data on Roche's drug Tecentriq, used to treat advanced squamous nonsmall cell lung cancer, was also presented in a media briefing over the weekend. It showed that adding Tecentriq to chemotherapy reduced patients' risk of death, or their disease worsening, by 29 percent versus chemotherapy alone.
Bristol's chief scientific officer, Thomas Lynch, recently referred to the Merck and Bristol medicines as Coke and Pepsi: More similar than different when it comes to treating lung cancer.
After the results from this weekend? "All hail the king," wrote investor Brad Loncar on Twitter, referring to Merck.
Loxo does it again
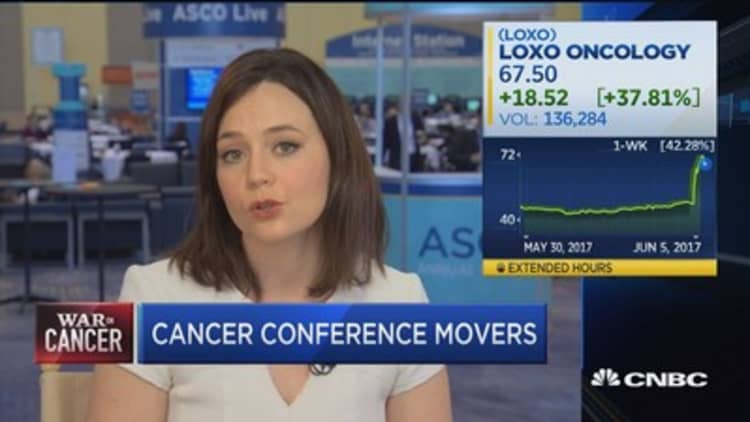
Young biotech Loxo Oncology was the belle of ASCO last year with data on its targeted medicine larotrectinib, which showed dramatic benefit for patients with rare cancers. That drug is now before the FDA for review.
This year, the company is back with the same strategy on another target, called RET fusions, which Loxo says are found in 2 percent of nonsmall cell lung cancer, and 10 to 20 percent of papillary and other thyroid cancers.
In a study of 82 patients, 77 percent of patients with RET-fusion cancers showed a response — meaning their tumors shrank — after taking Loxo's drug LOXO-292. In patients with RET-mutated medullary thyroid cancer, the response rate was 45 percent.
Six weeks ago, Loxo competitor Blueprint Medicines reported results for its drug BLU-667 on the same target. In nonsmall-cell lung cancer, the response rate was 50 percent; in medullary thyroid cancer, it was 40 percent.
High expectations for Bluebird and Celgene
Some of the first data most anticipated by Wall Street that emerged from ASCO were presented Friday evening on Celgene and Bluebird Bio's personalized immunotherapy for multiple myeloma. The treatment is a CAR-T — for chimeric antigen receptor T-cell therapy. It involves removing a patient's T-cells, genetically modifying them to identify a target on cancer cells (in this case, one called BCMA), and then giving them back to the patient.
Expectations were high running into the conference: Evercore ISI pegged them at an extension of patients' time without their cancer progressing (a measure known as PFS, for progression-free survival) of 12 to 15 months.
The data came in with a PFS of 11.8 months. Though some investors may be disappointed with the data, Bluebird CEO Nick Leschly expressed excitement, noting in an interview Saturday that patients in the trial had already tried a median of seven other therapies that had stopped working for them. Analysts were also supportive of the data.
"We think data looks quite good, especially in very sick population, and supports blockbuster potential for Celgene," Jefferies analyst Michael Yee wrote in a research note. Bluebird and Celgene are partners.
Nektar and Bristol advance partnered drug, after head-scratching data
Nektar signed a $1.85 billion deal with Bristol in February to partner on its drug NKTR-214, which the companies hope will improve the efficacy of Bristol's immunotherapy drug Opdivo.
However, results of a study of those drugs in combination reported Saturday were confusing. That's because they include patients in different stages of the trial: For example, in melanoma, 11 of 13 patients in the first stage of the trial responded to the combination therapy. But in stage 2, which had a shorter follow-up time, just 14 of 28 did.
Investors will have questions about why response rates declined from stage 1 to stage 2, but Bristol-Myers' head of oncology development, Dr. Fouad Namouni, called the results "encouraging." The companies are now planning to move the combination into a larger, confirmatory trial with hopes the results will support regulatory approval.
This should spare a lot more folks from chemo that won't really have much benefit.Doug Schenkel.Cowen analyst
A win for personalized medicine
It's hard to find something these days that doesn't fall into the category of personalized medicine, with all of the aforementioned cancer drug trials characterizing patients based on what's driving their disease. But one of the major pieces of news out of the weekend applies a personalized medicine approach to an old therapy: chemotherapy for breast cancer.
For a type of breast cancer that accounts for half of all cases, it hasn't been clear whether certain women benefited from chemotherapy, in addition to hormone therapy, after surgery. A study funded by the National Cancer Institute as well as other groups found that chemotherapy in this setting benefits about 30 percent of women: Another 70 percent are likely to see the same outcome without chemotherapy.
"Practically speaking, this means that thousands of women will be able to avoid chemotherapy, with all of its side effects, while still achieving excellent long-term outcomes," ASCO expert Burstein said in a press release about the trial, which is dubbed TAILORx.
The results may be a boon for the maker of the diagnostic test used in the trial, Genomic Health, said Cowen analyst Doug Schenkel.
"It should drive an increase in use," Schenkel wrote in an email to CNBC. "There were lots of questions about the utility of the test in patients who had intermediate oncotype results. This should spare a lot more folks from chemo that won't really have much benefit."

